Innovations in MedTech to be presented at the 32nd MTi Forum
Companies developing complex disease-solving data curation, heart-failure telemonitoringand home sperm testing will exclusively present in-person as Campden Wealth returns to Lausanne, Switzerland, for the 32nd edition of the MedTech Investing Europe Forum (MTi) on July 12 and 13.
MTi is Europe’s longest-running healthcare investment and partnering event. This year, the forum will showcase up to 40 of the latest investment opportunities across devices, healthcare, life sciences and digital health technology.
Chaired by Professor Benoît Dubuis, executive director of Campus Biotech and president of the Inartis Foundation, the forum will welcome an audience of active investors including venture capitalists, private equity funds, family offices, private wealth holders, major healthcare corporations, angel investors and more.
The conference programmes are developed in conjunction with the MTi Investor Advisory Board. Critical topics exploring established, nascent and anticipated evolutions of wellbeing and health monitoring, diagnostics, treatments, regulations, scaling and business structures are debated with experienced leaders and investors.
The presenting companies, of which three are highlighted below, represents the truly global nature of the healthcare and life-science venture ecosystem, with leading organisations from Europe, North America, the Middle East, and Asia.

Qureight
Founded in 2018, the UK-based Qureight is the brainchild of senior physician Dr. Muhunthan Thillai and radiologist Dr. Alessandro Ruggiero (pictured above), with a mission to use pioneering data curation to solve complex diseases.
Using cloud-based platform technology, Qureight structures images, biomarkers, and clinical data in conditions such as Idiopathic Pulmonary Fibrosis (IPF), pulmonary hypertension and lung cancer.
Qureight partners with healthcare providers to structure real world data and supercharge their machine-learning models. As a result, the company can speed up lengthy clinical trials and find new study endpoints.
“A lot of companies look at CT scans to produce radiomic features in diseases. We have developed a platform technology which uses machine-learning pathways to combine multiple data sources to produce new endpoints,” says Dr. Muhunthan Thillai of the company will be publishing results with key pharma partners at two international conferences in September 2022.
Aiming for ISO certification by August 2022, Qureight’s first patented products include automated methods to identify changes in lung airways and blood vessels in response to disease progression and novel drug development.
Qureight acquired £2 million in seed funding in December, 2021, and has used the money to build the technical team and begin rapid commercial partnerships with biopharma clients including AstraZeneca, Takeda and Merck. Qureight is now considering the next round of funding to capitalise on the recent developments and accelerate platform development.

Heartfelt Technologies
Positioned as the future of heart-failure telemonitoring, Heartfelt Technologies was founded in 2015 by three Cambridge graduates, seeking to solve the problem of patient noncompliance in healthcare.
“The idea for this start-up business began with a random conversation with a heart transplant surgeon at a party in 2015,” says Heartfelt Technologies’ Chief Scientific Officer Dr Oriane Chausiaux. “‘Do you know,’ he said, ‘That half the people I operate on wouldn’t require surgery at all if they reported their symptoms.’
“The founders of Heartfelt Technologies went away and checked the facts and, in so doing, unearthed the often-hidden problem of ‘non-adherent’ patients. These are mostly elderly people, often living alone, many with deteriorating mental capacity, who are unable to follow the advice given to them by their doctors. Instead of calling for help as their symptoms get worse, they end up as emergency hospital admissions. The medical industry is very good at developing new technologies to help the kinds of patients who enrol in clinical trials, but because the vast majority of clinical trials excludes these patients, most medical devices fail in this most vulnerable patient group.”
The Heartfelt device solves patient compliance in heart failure in a unique way: by ignoring it. The design is currently focused on patients with dementia and those who persistently fail to adhere to their self-care plans (medicines, self-monitoring, reporting etc). This patient group is relatively small but accounts for the vast majority of hospitalisations and, therefore, health system costs.
The Heartfelt device is a small 3D sensor, which passively monitors patients as they go about their daily lives and detects the symptoms of impending heart failure.
The Heartfelt device has undergone multiple clinical trials and pilots, with more than 180 devices installed in patient homes.
The founding team have recently exited their previous remote patient monitoring start-up, DuoFertility, following that product being taken on by Boots PLC and investment by a private equity firm.
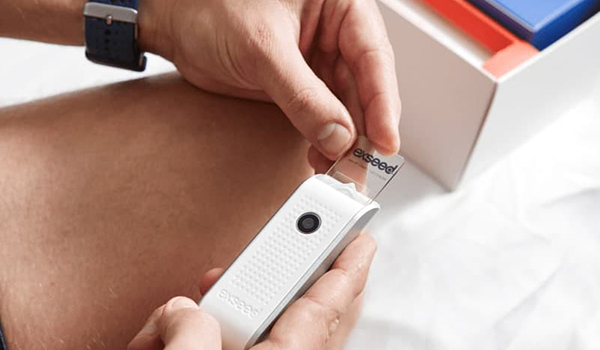
ExSeed Health
Innovating home sperm testing with clinical-grade results, ExSeed Health is a UK/Danish MedTech/Software company specialising in male reproductive health.
Founded in 2017 by Morten G. Ulsted and Emil Andersen, ExSeed has developed fast-result, state-of-the-art medical devices enabling highly accurate and easy at-home and in-clinic testing of male fertility to empower patients and clinicians. The innovative device enables men to see their sperm cells in real-time via a high-resolution video, which is recorded via an app and sent to the cloud for instant analysis with more than 95% accuracy versus advanced laboratory equipment.
“ExSeed’s point-of-care diagnostics platform works to democratise access to healthcare, both for patients and clinics, by providing the same level of a diagnostics accuracy as expensive lab-based systems costing more than 600 times the price of one of ExSeed’s devices,” says chief executive officer and co-founder Morten G. Ulsted. “ExSeed’s software as a service (SaaS) lifestyle programme has further shown great efficacy in optimising chances of conception. Studies show that around 50% of the men undergoing ExSeed’s lifestyle program are able to move out of a low sperm-quality category, leading to greatly improved chances of reaching pregnancy with a partner.”
Post-results, the app uses AI to suggest personalised and scientifically researched advice to help improve sperm health. The remote medical team review results and offer guidance as a part of the service for high-level patient care and support. A medical report is automatically generated, which can be shared with a fertility clinic or doctor.
The system is CE certified as an IVD Medical Device in Europe and on its way to becoming FDA approved.
For further information, contact Peter Newton via peternewton@campdenwealth.com.
Campden Wealth is not an investment advisory service and is not a registered investment adviser or broker/dealer and has performed no due diligence and does not endorse any investment services, strategies or managers listed in Managerlink or at any other Campden Wealth event. You should perform your own extensive due diligence.






