Introducing the medical device firm that is bringing catheters into the 21st Century

Catheters have been used to facilitate urinary flow for more than 3,000 years. There is evidence that the Ancient Greeks used straw reeds to relieve urinary retention. Gold and silver tubes were used through the 1800s because of their high ductility. Progress has been very slow and devices in use today are still messy, uncomfortable and prone to infection. The team at Ingenion Medical Limited (“Ingenion” or the "Company”), a privately-held, medical device company headquartered in the UK, is poised to solve this ancient problem with the cymactive™ 2.0R bladder management system, a more comfortable and better-performing alternative.
Discover more about the evolution of cymactive™ 2.0R and its huge potential…
Can you talk about the evolution of the cymactive bladder management system?
The first-generation device was invented by Phillip Davis, a mechanical engineer. After graduating from the Massachusetts Institute of Technology (MIT), Phil adapted computers and work settings to facilitate jobs for the blind. While on a dream vacation to a South Pacific island with his family, Phil suffered a fall and fractured the fifth vertebra from the base of his spine, paralyzing some leg muscles and leaving him unable to urinate, a condition called urinary retention. Fortunately, a series of operations have preserved Phil’s ability to walk with a cane. While he still cannot feel changes in what he step on and lives with a chronic low level of leg pain, urinary retention remains his most difficult and limiting problem.
At first, Phil used Foley catheters, which came with constant discomfort, leg bags and risk of infection. He soon learned that he could discard the Foley leg bag by keeping the catheter’s distal end plugged between urinations. Later, he discarded the indwelling Foley entirely and changed to catheterizing himself several times a day using a simple straight catheter. He found this vastly preferable, but it required a steady supply of single-use catheters. Phil became determined to design a better device for himself and for others who cope with urinary retention.
How did you identify the need and develop the invention?
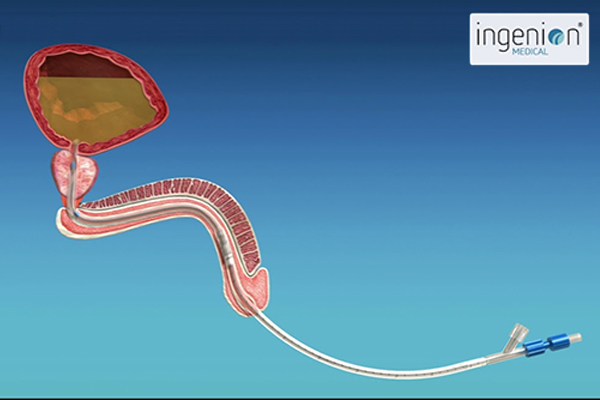
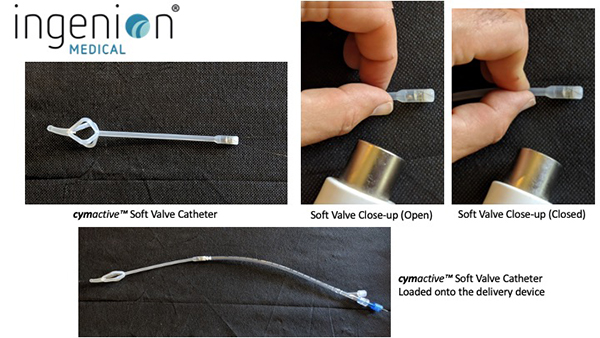
As an engineer, Phil realized that his urinary retention was essentially a mechanical problem. He designed an indwelling catheter with a tiny valve that could be inserted into the urethra and replaced each month. When needed, the valve could be opened by holding a small magnet against the body where the valve was located, and closed when the hand-held magnet was withdrawn. A nylon string used to remove the device was all that protruded from the urethra. After Phil built a prototype and patented it, he had to face the challenge of selling it or developing a company. He turned to his brother-in-law, Dr. Sergio Rothstein, a clinical psychologist and successful businessman, and Sergio’s cousin, Raphael Wurzel, an experienced urologist. Sergio would go on to fund the original company which was called Urovalve, Inc., while Rafi contributed important insights into the medical challenges that Phil’s device would need to overcome.
As one of the four founders of Urovalve, Sergio led the formation of Ingenion Medical Limited in 2013. He is currently the Chairman of the Board. The first Ingenion device (known originally as CymActive) was safety-tested with approximately 40 patients in three countries (USA, UK and Argentina). CymActive achieved CE Mark authorisation under the EU’s old MDD (Medical Device Directive) regulatory regime. From that experience, the Company made design improvements that are expected to increase both the comfort and performance of the catheter. The new, improved cymactive™ 2.0R device is designed for men with chronic, non-neurogenic urinary retention. Additional devices patented by the Company have the potential to treat chronic incontinence for both women and men. Together, these devices represent a multi-billion-dollar global market opportunity.
Ingenion has fully developed the cymactive™ 2.0R device and is planning to launch it commercially once it has been CE Marked under the EU’s new MDR system. Ingenion is also planning to apply for US 501k approval of thecymactive™ 2.0R device some time in 2024.
“With cymactive™ 2.0R close to entering the European market, it's an exciting time.” said Dr. Rothstein. “The product is poised to disrupt old technology and improve the lives of many, many patients.”
How is the cymactive™ 2.0R device a major improvement over current methods?
The urethra is an important part of the human body’s natural defense system against infection. Most of the time, the urethra is a flat tube with a mucosal membrane, but bacteria can migrate along the outer surface of an indwelling catheter and up to the bladder where a traditional catheter creates a permanent reservoir of urine inside the bladder where bacteria can breed. Likewise, repeatedly inserting intermittent catheters each day can increase incidences of infection and disturbances to the mucosal membrane.
Today, intermittent catheterization and the Foley catheter are the most prevalent methods for treating urinary control issues. The Foley was first developed almost 100 years ago when most medical patients were bed-bound in hospital environments.
Catheter Associated Urinary Tract Infections (CAUTIs) are a major cost to every country’s healthcare system. For example, in the UK alone, CAUTIs cost the National Health Service between £1 – £2.5 billon and result in more than 2,100 excess deaths per year. One of the market leaders in the catheter industry, Coloplast A/S, estimates that long-term catheter users experience 2.7 CAUTIs each year. The average cost to treat them is £3,500 which adds approximately £9,450 to the annual cost of catheters or nearly £800 per month.
Ingenion’s cymactive™ 2.0R can be used for up to 30 days, replacing as many as 200 intermittent catheters, and omitting considerable medical waste and packaging. Targeting long-term catheter users will create a very attractive recurring revenue stream.

How has the solution been received so far by medical practitioners and patients?
Virtually every clinician and patient who has seen Ingenion’s cymactive™ 2.0R catheter has been anxious to try it. Importantly, this includes urological nurses, the people most often dealing with blocked Foley catheters and CAUTIs.
The intuitive nature of the design is very attractive, and the elimination of external leg bags gives patients increased control and dignity. They are also expected to experience lower incidence of infections than traditional methods. Discussions with potential patients have revealed advantages that were not anticipated. For example, one long-term user of the Foley catheter who was of the Islamic faith explained that having an external bag of urine strapped to his leg was a point of shame during his daily prayer sessions. Ingenion’s device would allow people who need urinary control to have a level of discretion and dignity that is just not possible with traditional Foley catheters.
Does Ingenion Medical Limited have any other innovations in the pipeline?
In late 2019, Ed Cappabianca was brought into the Company as the new CEO to help bring the second generation of our lead device to the market. Although progress during the global pandemic was challenging, support from the Board and our major shareholders was excellent. In addition, from 2020 to early this year, Ingenion was successful in winning three UK government grants totaling £440,000. These supplemented the approximately £1.5 million in equity raised from new and existing investors in the UK and USA during that period.
The grants and equity funding assisted in the completion of our lead device, the cymactive™ 2.0R, as well as the initial development of our next two devices to treat chronic incontinence for women and men. Non-dilutive capital from grants has helped to accelerate Ingenion’s product development pipeline and expand our growing patent portfolio which currently includes three granted patents and three new patent applications. Two of the new products in the pipeline are expected to be available as soon as early 2025, subject to raising additional funding, some of which may come from future grants.
Since 2022, Ingenion’s dynamic team has grown. Our chief operating officer, Susan Finch, has significant experience in medical device manufacturing. Tim Maloney, our commercial director, has led teams introducing complex medical devices in more than 15 different countries, and worked closely with distributors and clinicians to build awareness and adoption. James Barnett leads our in-house engineering design activities, after more than 13 years in a similar role for a division of Johnson & Johnson. Our chief financial officer, Zia Mursaleen, has been with Ingenion since 2017 and his background as a chartered accountant includes more than ten years with Deloitte.
Where are you currently on the path to launch?
The Company achieved ISO 13485 certification last year, an essential step on the path to regulatory approval. Although the current product is not yet commercially available, Ingenion has submitted its CE Mark application for thecymactive™ 2.0R as a Class IIb medical device and expects approval in 2023. Ingenion is in commercial discussions with multiple medical device distribution companies across Europe who are interested in the product and have established relationships in the urology sector.
“I am looking forward to building a distributor network to make cymactive™’s benefits available to as many patients as possible,” said commercial director Tim Maloney. “cymactive™ is one of those special products capable of significantly improving the mobility and quality of life for patients who need it. It is a joy and privilege to work on.”

For more information on Ingenion Medical Limited, click here.
Campden Wealth is not an investment advisory service, is not a registered investment adviser or broker/dealer, and has performed no due diligence. It does not endorse any investment services, strategies or managers listed in Managerlink or at any other Campden Wealth event. You should perform your own extensive due diligence.






